Every living organism on Earth depends on incredibly small molecules that carry enormous biological responsibility. Specifically, nucleotides serve as the foundational building blocks that construct DNA, RNA, and critical energy-carrying molecules within every cell. Furthermore, these remarkable structures participate in nearly every major biological process that keeps living systems functioning properly. Therefore, understanding nucleotides unlocks a deeper appreciation for how life stores information, transfers energy, and perpetuates itself across generations.
What Exactly Are Nucleotides?
A Clear Scientific Definition
Nucleotides represent the monomeric units that polymerize together to form nucleic acids like DNA and RNA. Additionally, each individual unit carries three distinct chemical components that work together to fulfill specific biological functions. Moreover, cells synthesize these molecules through complex metabolic pathways that scientists have studied extensively over many decades. Consequently, modern biochemistry treats these structures as absolutely central to understanding how living systems operate at the molecular level.
The Three Core Components
Every nucleotide contains three essential chemical components joined together through precise covalent bonding arrangements. Furthermore, a nitrogenous base forms the information-carrying portion that distinguishes one nucleotide type from another. Additionally, a five-carbon sugar molecule provides the structural backbone to which the other components attach securely. Moreover, one or more phosphate groups complete the structure and contribute significant chemical energy to biological reactions. Therefore, removing any single component destroys the molecule’s ability to fulfill its biological functions effectively.
Nucleosides Versus Nucleotides
Scientists sometimes use the term nucleoside, which describes a nitrogenous base attached to a sugar without any phosphate group. Furthermore, adding one or more phosphate groups to a nucleoside creates the complete nucleotide structure with full biological activity. Additionally, this distinction matters practically because cells process nucleosides and nucleotides through different metabolic pathways. Consequently, understanding the difference helps students and researchers interpret biochemical literature with greater accuracy and confidence.
The Structural Components in Detail
Nitrogenous Bases: The Information Carriers
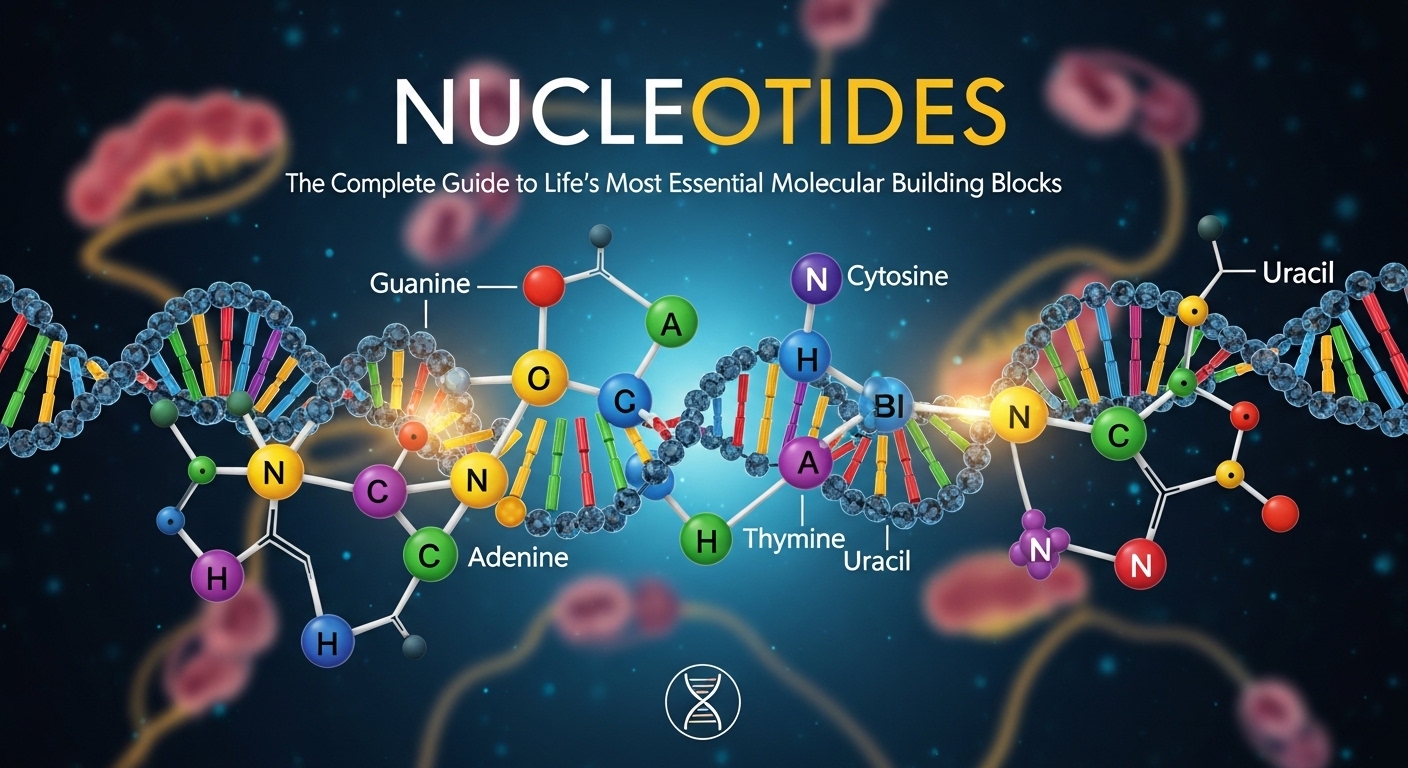
Nitrogenous bases represent the chemically unique components that give each nucleotide its distinct identity and function. Moreover, chemists classify these bases into two major structural categories based on their ring structure and chemical properties. Furthermore, purines contain a double-ring structure and include adenine and guanine as their primary biological representatives. Additionally, pyrimidines carry a single-ring structure and include cytosine, thymine, and uracil as their main biological members. Consequently, the specific sequence of these bases along a nucleic acid strand encodes all genetic information.
Adenine and Its Biological Significance
Adenine pairs specifically with thymine in DNA and with uracil in RNA through precise hydrogen bonding interactions. Furthermore, this base also forms part of adenosine triphosphate, the primary energy currency that powers cellular metabolism. Additionally, adenine appears in coenzymes like NAD and FAD that drive essential metabolic reactions throughout the cell. Therefore, adenine ranks among the most biologically versatile and metabolically important nitrogenous bases in living systems.
Guanine and Its Unique Properties
Guanine pairs specifically with cytosine through three hydrogen bonds, creating the strongest base pair in nucleic acids. Moreover, this triple bonding arrangement contributes significantly to the structural stability of DNA double helix regions. Furthermore, guanine-rich sequences can form specialized four-stranded structures called G-quadruplexes with important regulatory functions. Consequently, researchers actively study guanine chemistry as a target for developing new cancer therapies and antiviral medications.
Cytosine, Thymine, and Uracil
Cytosine pairs with guanine in both DNA and RNA, contributing to structural integrity throughout nucleic acid molecules. Additionally, thymine appears exclusively in DNA, where it pairs with adenine through two stabilizing hydrogen bonds. Moreover, uracil replaces thymine in RNA molecules, differing structurally by the absence of a methyl group. Furthermore, this difference allows cells to distinguish RNA from DNA, which matters enormously for proper cellular information processing. Therefore, even subtle structural variations between bases carry significant functional consequences at the cellular level.
The Five-Carbon Sugar Component
The sugar component determines whether a nucleotide belongs to DNA or RNA through one critical structural difference. Moreover, deoxyribose sugar contains one fewer oxygen atom than ribose, which gives DNA its characteristic chemical stability. Furthermore, ribose sugar’s extra hydroxyl group makes RNA more chemically reactive and structurally flexible than DNA. Additionally, this difference in sugar chemistry explains why DNA stores genetic information long-term while RNA performs more transient cellular functions. Consequently, the seemingly minor sugar difference produces profoundly different biological roles for these two nucleic acid types.
Phosphate Groups and Chemical Energy
Phosphate groups attach to the sugar component and contribute both structural connectivity and chemical energy to nucleotides. Furthermore, the bonds connecting multiple phosphate groups store substantial chemical energy that cells release during metabolic reactions. Additionally, hydrolysis of these high-energy bonds powers muscle contraction, biosynthesis, and active transport across cell membranes. Moreover, cells carefully regulate phosphate group addition and removal to control when and where biological energy becomes available. Therefore, phosphate chemistry sits at the very heart of cellular energy management and metabolic regulation.
Types of Nucleotides and Their Classification
DNA Nucleotides
DNA nucleotides incorporate deoxyribose sugar and one of four nitrogenous bases: adenine, guanine, cytosine, or thymine. Additionally, cells link these four types together in specific sequences that encode every piece of genetic information. Moreover, the precise sequence of DNA nucleotides determines which proteins a cell produces and when it produces them. Furthermore, mutations that alter this sequence can produce profound effects ranging from beneficial adaptations to serious genetic diseases. Consequently, DNA nucleotide sequence integrity represents one of the cell’s most carefully protected biological assets.
RNA Nucleotides
RNA nucleotides incorporate ribose sugar and use uracil instead of thymine as their fourth nitrogenous base type. Moreover, cells produce several distinct RNA types that perform different functions throughout the gene expression process. Furthermore, messenger RNA carries genetic instructions from the nucleus to ribosomes where protein synthesis occurs. Additionally, transfer RNA and ribosomal RNA perform essential structural and catalytic roles during the translation process itself. Therefore, RNA nucleotides collectively enable the conversion of genetic information into functional protein molecules.
Energy-Carrying Nucleotides
Certain nucleotides serve primarily as energy carriers rather than as structural components of nucleic acids. Moreover, adenosine triphosphate contains three phosphate groups and releases energy when enzymatic hydrolysis breaks phosphate bonds. Furthermore, guanosine triphosphate powers specific cellular processes including protein synthesis and signal transduction pathways. Additionally, cytidine triphosphate plays essential roles in lipid metabolism and membrane synthesis throughout the cell. Consequently, energy-carrying nucleotides function as the cell’s primary rechargeable batteries powering virtually every biological activity.
Coenzyme Nucleotides
Several crucial cellular coenzymes contain nucleotide components that enable their electron-carrying biochemical functions. Furthermore, nicotinamide adenine dinucleotide contains an adenine nucleotide and participates in hundreds of oxidation-reduction reactions. Additionally, flavin adenine dinucleotide similarly carries electrons through the mitochondrial electron transport chain efficiently. Moreover, coenzyme A contains a nucleotide component and plays a central role in fatty acid metabolism and the citric acid cycle. Therefore, nucleotide-containing coenzymes extend biological influence far beyond their roles in nucleic acid structure.
How Cells Build Nucleotides
De Novo Synthesis Pathways
Cells construct nucleotides from scratch through complex metabolic pathways called de novo synthesis routes. Furthermore, purine synthesis begins with ribose-5-phosphate and builds the ring structure through a series of enzymatic steps. Additionally, pyrimidine synthesis assembles the ring structure first before attaching it to the ribose sugar component. Moreover, these biosynthetic pathways require significant metabolic investment in terms of energy and raw material consumption. Consequently, cells carefully regulate de novo synthesis to match nucleotide production with actual cellular demand.
Salvage Pathways
Cells also recover and recycle nucleotide components through metabolic routes called salvage pathways. Moreover, these pathways capture free bases and nucleosides released during nucleic acid degradation and reincorporate them efficiently. Furthermore, salvage pathways consume far less energy than de novo synthesis, making them metabolically economical alternatives. Additionally, certain cell types including red blood cells rely almost exclusively on salvage pathways because they lack de novo synthesis enzymes. Therefore, salvage pathways represent an elegant evolutionary solution to the metabolic cost of nucleotide production.
Regulation of Nucleotide Synthesis
Cells regulate nucleotide synthesis through feedback inhibition mechanisms that sense and respond to current nucleotide concentrations. Furthermore, high concentrations of end products inhibit the enzymes that catalyze early steps in the biosynthetic pathway. Additionally, this regulatory system prevents wasteful overproduction while ensuring adequate nucleotide availability for DNA replication and RNA synthesis. Moreover, disrupting these regulatory mechanisms contributes to the uncontrolled cell proliferation that characterizes cancer development. Consequently, nucleotide synthesis regulation represents an important target for anti-cancer drug development research.
The Role of Nucleotides in DNA Structure
Forming the Double Helix
Nucleotides link together through phosphodiester bonds to form long polynucleotide chains that constitute DNA strands. Furthermore, two complementary strands wind around each other to form the iconic double helix structure Watson and Crick described. Additionally, base pairing between complementary strands holds the two chains together through specific hydrogen bonding interactions. Moreover, the sugar-phosphate backbone faces outward while the bases point inward toward the helix’s central axis. Consequently, this architecture simultaneously protects the information-carrying bases and allows precise enzymatic access during replication.
Base Pairing Rules and Genetic Fidelity
Adenine pairs exclusively with thymine while guanine pairs exclusively with cytosine in DNA double-stranded structures. Furthermore, these specific pairing rules ensure that DNA replication produces accurate copies of the original genetic information. Additionally, DNA repair enzymes use complementary base pairing to identify and correct replication errors before they become permanent. Moreover, the predictability of base pairing allows scientists to design precise molecular tools like probes and primers. Therefore, the strict base pairing rules that govern nucleotide interactions underpin the entire fidelity of genetic inheritance.
DNA Replication and Nucleotide Demand
Dividing cells must duplicate their entire DNA content, consuming enormous quantities of nucleotides in the process. Furthermore, the replication machinery incorporates nucleotides into new strands at remarkable speed while maintaining high accuracy. Additionally, insufficient nucleotide availability during S phase forces cells to pause replication and activate checkpoint responses. Moreover, chemotherapy drugs that interfere with nucleotide availability exploit this dependency to halt cancer cell proliferation. Consequently, understanding nucleotide demand during replication informs both cancer biology and drug development strategies.
The Role of Nucleotides in RNA and Protein Synthesis
Transcription: Copying Genetic Information
During transcription, RNA polymerase enzymes use DNA as a template to synthesize complementary RNA molecules. Furthermore, the enzyme incorporates ribonucleotides one by one according to the base pairing rules governing complementary strand synthesis. Additionally, the resulting messenger RNA molecule carries a copy of the gene’s information from the nucleus to ribosomes. Moreover, the accuracy of nucleotide incorporation during transcription directly determines the fidelity of protein synthesis downstream. Therefore, transcription represents the critical first step in converting nucleotide sequences into functional biological molecules.
Translation: From Nucleotide Code to Protein
Ribosomes read messenger RNA sequences in three-nucleotide units called codons during the translation process. Furthermore, each codon specifies a particular amino acid that transfer RNA molecules deliver to the growing protein chain. Additionally, the genetic code that maps codons to amino acids exhibits redundancy that provides some protection against mutation effects. Moreover, the start and stop codons encoded within RNA nucleotide sequences precisely define the boundaries of each protein. Consequently, the nucleotide sequence of a gene ultimately determines the complete amino acid sequence of the resulting protein.
Nucleotides as Cellular Signaling Molecules
Cyclic AMP as a Second Messenger
Adenosine monophosphate can form a cyclic structure called cyclic AMP that serves as an important intracellular signal. Furthermore, the enzyme adenylyl cyclase produces cyclic AMP in response to hormones binding cell surface receptors. Additionally, cyclic AMP activates protein kinase A, which phosphorylates target proteins to alter their activity and function. Moreover, cells rapidly degrade cyclic AMP through phosphodiesterase enzymes to terminate signaling precisely when appropriate. Therefore, this nucleotide derivative plays a central role in hormonal signal transduction throughout the body.
Cyclic GMP and Physiological Regulation
Cyclic GMP similarly functions as a second messenger in numerous important physiological regulatory pathways. Furthermore, this molecule mediates smooth muscle relaxation, visual signal transduction, and kidney function regulation. Additionally, the drug sildenafil works by inhibiting the phosphodiesterase enzyme that degrades cyclic GMP in smooth muscle. Moreover, nitric oxide signaling in blood vessels operates largely through cyclic GMP-dependent mechanisms. Consequently, nucleotide-based signaling molecules influence physiology in ways that extend from vision to cardiovascular function.
Purinergic Signaling
Cells release nucleotides like ATP into the extracellular space where they act on purinergic receptors on neighboring cells. Moreover, this purinergic signaling system regulates diverse functions including inflammation, pain perception, and vascular tone. Furthermore, platelets release ATP and ADP during blood vessel injury to coordinate the clotting response effectively. Additionally, purinergic signaling in the nervous system modulates neurotransmission and contributes to chronic pain development. Therefore, extracellular nucleotide signaling represents a sophisticated communication system that coordinates tissue-level physiological responses.
Nucleotides in Human Health and Disease
Genetic Mutations and Disease
Errors in nucleotide sequence, called mutations, underlie a vast array of human genetic diseases and cancers. Furthermore, point mutations involving single nucleotide changes can dramatically alter protein function with serious health consequences. Additionally, insertion or deletion mutations disrupt the reading frame and typically produce nonfunctional protein products. Moreover, accumulation of somatic mutations in oncogenes and tumor suppressor genes drives cancer development over time. Consequently, understanding nucleotide sequence integrity and mutation mechanisms remains central to modern medical genetics.
Nucleotide Metabolism Disorders
Defects in nucleotide metabolism enzymes cause rare but serious inherited diseases with diverse clinical presentations. Furthermore, adenosine deaminase deficiency disrupts purine metabolism and causes severe combined immunodeficiency disease in affected children. Additionally, Lesch-Nyhan syndrome results from hypoxanthine-guanine phosphoribosyltransferase deficiency and causes severe neurological dysfunction. Moreover, gout develops when excessive uric acid, a purine metabolism end product, crystallizes in joints causing painful inflammation. Therefore, nucleotide metabolism disorders illustrate how fundamentally these molecules influence human health across multiple organ systems.
Antiviral and Anticancer Nucleotide Analogues
Pharmaceutical researchers have developed nucleotide analogues that interfere with viral replication or cancer cell proliferation. Furthermore, drugs like acyclovir mimic natural nucleotides but terminate viral DNA chain elongation when incorporated into viral genomes. Additionally, nucleoside reverse transcriptase inhibitors form the backbone of antiretroviral therapy for HIV infection management. Moreover, anticancer drugs like 5-fluorouracil and gemcitabine disrupt nucleotide metabolism in rapidly dividing tumor cells. Consequently, nucleotide chemistry has provided some of medicine’s most important and widely used therapeutic agents.
Nucleotides in Nutritional Science
Dietary nucleotides contribute to gastrointestinal health, immune function, and tissue repair under various physiological conditions. Furthermore, infant formula manufacturers add nucleotides to approximate the nucleotide content naturally present in human breast milk. Additionally, research suggests dietary nucleotides support intestinal mucosal development and enhance immune responses in young children. Moreover, athletes and critically ill patients may benefit from nucleotide supplementation during periods of high cellular demand. Therefore, nutritional science increasingly recognizes nucleotides as conditionally essential dietary components beyond their classical roles.
Nucleotides in Modern Biotechnology
DNA Sequencing Technologies
Modern DNA sequencing technologies rely on controlled nucleotide incorporation to determine the precise sequence of genetic material. Furthermore, next-generation sequencing platforms process billions of nucleotide incorporation events simultaneously to sequence entire genomes rapidly. Additionally, fluorescently labeled nucleotide analogues allow sequencing instruments to optically identify each incorporated base. Moreover, falling sequencing costs driven by nucleotide chemistry innovations have revolutionized genomics, medicine, and evolutionary biology research. Consequently, nucleotide chemistry innovations continue driving transformative advances across the entire life science research enterprise.
PCR and Molecular Diagnostics
Polymerase chain reaction technology amplifies specific DNA sequences by repeatedly incorporating nucleotides through enzymatic synthesis. Furthermore, PCR requires carefully optimized nucleotide concentrations to achieve efficient and specific amplification of target sequences. Additionally, diagnostic PCR tests detect viral and bacterial pathogens by amplifying pathogen-specific nucleotide sequences from patient samples. Moreover, COVID-19 diagnostic testing relied entirely on PCR-based nucleotide detection technology during the global pandemic. Therefore, nucleotide chemistry underpins diagnostic technologies that directly impact clinical medicine and public health outcomes.
Gene Editing With CRISPR
CRISPR-Cas9 gene editing technology achieves precision by targeting specific nucleotide sequences within the genome. Furthermore, guide RNA molecules containing complementary nucleotide sequences direct the Cas9 enzyme to precise genomic locations. Additionally, researchers can use CRISPR to correct disease-causing nucleotide mutations with unprecedented precision and efficiency. Moreover, therapeutic applications targeting genetic diseases caused by specific nucleotide errors represent an exciting emerging medical frontier. Consequently, nucleotide sequence specificity drives the precision that makes CRISPR such a revolutionary tool in modern biology.
Synthetic Biology Applications
Synthetic biologists design artificial nucleotide sequences that encode novel proteins, regulatory elements, and metabolic pathways. Furthermore, some research groups have expanded the genetic alphabet by creating unnatural nucleotide base pairs. Additionally, these expanded genetic codes allow cells to incorporate non-natural amino acids into proteins with unique chemical properties. Moreover, synthetic nucleotide sequences power applications ranging from biosensors to biofuels and pharmaceutical protein production. Therefore, nucleotide chemistry innovations continue opening entirely new frontiers in both basic research and applied biotechnology.
How Diet and Lifestyle Affect Nucleotide Metabolism
Foods Rich in Nucleotides
Animal proteins, organ meats, and certain seafoods provide dietary purines that the body converts through metabolic pathways. Furthermore, foods like liver, anchovies, sardines, and mussels contain particularly high concentrations of purine-rich nucleotides. Additionally, plant-based foods including legumes and whole grains contribute moderate nucleotide and nucleoside content to the diet. Moreover, fermented foods and yeast extracts contain substantial nucleotide concentrations that influence cellular metabolism. Consequently, dietary choices significantly influence the metabolic load on nucleotide synthesis and degradation pathways.
Hydration and Nucleotide Clearance
Adequate hydration supports efficient kidney clearance of uric acid and other nucleotide metabolism end products. Furthermore, dehydration concentrates uric acid in the blood, increasing the risk of crystal formation in joints. Additionally, high fluid intake reduces gout flare frequency in susceptible individuals by maintaining urate solubility. Moreover, coffee consumption associates with lower gout risk, possibly through effects on renal urate excretion. Therefore, simple lifestyle choices around fluid intake meaningfully influence nucleotide metabolism outcomes in everyday life.
Exercise and Nucleotide Turnover
Intense physical exercise dramatically increases nucleotide turnover in muscle cells through elevated ATP consumption and regeneration. Furthermore, adenylate kinase and AMP deaminase enzymes manage nucleotide ratios during the metabolic stress of intense exercise. Additionally, post-exercise recovery requires active nucleotide resynthesis to restore depleted muscle energy reserves completely. Moreover, endurance training adaptations include enhanced mitochondrial nucleotide regeneration capacity in trained muscle tissue. Consequently, regular exercise not only consumes nucleotides but also drives the metabolic adaptations that improve future nucleotide management.
Recent Advances in Nucleotide Research
mRNA Vaccine Technology
The COVID-19 mRNA vaccines demonstrated the therapeutic power of synthetic nucleotide sequences delivered directly into human cells. Furthermore, researchers chemically modified the nucleotides in vaccine mRNA to reduce immunogenicity and improve translation efficiency. Additionally, modified nucleotide chemistry allowed the mRNA to persist long enough to generate robust immune responses. Moreover, this nucleotide modification technology now drives development of mRNA vaccines against numerous other infectious diseases. Consequently, nucleotide chemistry innovations during the pandemic accelerated an entire new era of vaccine development technology.
Nucleotide-Based Therapeutics
Antisense oligonucleotides represent short synthetic nucleotide sequences that modulate gene expression with remarkable precision. Furthermore, FDA-approved antisense drugs now treat conditions including spinal muscular atrophy and certain forms of hereditary blindness. Additionally, small interfering RNA molecules use nucleotide complementarity to silence specific disease-causing genes in targeted tissues. Moreover, nucleotide aptamers function as molecular recognition tools that bind therapeutic targets with antibody-like specificity and affinity. Therefore, nucleotide-based therapeutics represent one of the most rapidly expanding areas in modern pharmaceutical development.
Epigenetic Nucleotide Modifications
Researchers have discovered that cells chemically modify nucleotides within DNA to regulate gene expression without changing sequence. Furthermore, DNA methylation adds a methyl group to cytosine nucleotides, typically silencing nearby gene expression. Additionally, hydroxymethylcytosine represents another modified nucleotide form with distinct regulatory functions in neuronal cells. Moreover, mapping these epigenetic nucleotide modifications across entire genomes reveals how environment influences gene expression patterns. Consequently, epigenetic nucleotide modifications bridge the gap between genetic sequence and environmental influence on biological outcomes.
Final Thoughts
Nucleotides stand as arguably the most consequential molecules in all of biology given their pervasive functional roles. Moreover, they simultaneously store genetic information, transfer cellular energy, carry signals, and enable the entire pharmaceutical industry. Furthermore, every major advance in modern medicine and biotechnology ultimately traces back to deeper understanding of nucleotide chemistry. Therefore, investing time in truly understanding these remarkable molecules rewards students, researchers, and curious minds with profound biological insight and appreciation.